The Challenge
About 37 million — or one in six(1,2) — American adults suffer from overactive bladder (OAB), while nearly 20 million experience fecal incontinence(3,4). A stigma exists around acknowledging these pelvic health issues. For more than 20 years, patients have been receiving symptom relief using the InterStim™ from Medtronic, but the company sought technology that would offer a discreet and user-friendly way to put control of the therapy in the patient’s hands.
The Solution
Medtronic wanted to replace its legacy patient programmer device, which had limited functionality and an obtrusive form factor, with user-friendly mobile devices that patients would be familiar with and would not draw attention to their condition. Through collaboration with Samsung, Medtronic developed a new InterStim™ smart programmer using a Galaxy smartphone and therapy applications. The smartphone is preloaded with Medtronic proprietary software applications that let patients discreetly manage their treatment.
The Results
With the new smart programmer, patients can now manage their InterStim™ implant using a familiar device. The smart programmer allows patients to adjust their therapy in front of others without drawing attention to the fact that they have a pelvic health condition, because it looks like a smartphone. The technology has now been adopted by new InterStim™ patients in the United States and Europe, providing a more user-friendly face to the therapy and putting control into the hands of patients.

Medtronic, a global healthcare solutions company, is headquartered in Dublin, Ireland and operates in 150+ countries. For nearly 60 years, the company’s mission has been to alleviate pain, restore health, and extend life for people around the world. The company’s 86,000 employees look to that mission as an ethical framework and inspirational goal as they use medical technologies, services and solutions to meet the needs of patients and healthcare professionals.
Healthcare faces many challenges, and Medtronic believes that many of those challenges can be solved with technology. The company is committed to partnering with others to improve and transform millions of lives each year through the use of its medical devices.
Learn more about how Medtronic is transforming healthcare
The Challenge
Finding a Discreet, Consumer-Centric Way to Treat Pelvic Health Issues
“For people with untreated overactive bladder or fecal incontinence, their world gets smaller and smaller,” explains Jim Willenbring, senior director for digital health and patient operations at Medtronic. “Their life starts to center on knowing the exact location of each bathroom between home and every place they frequent — work, school, shopping centers, the gym.”
Without treatment, the lives of people with these conditions can become tethered to bathroom locations, with many not wanting to leave home at all, suffering in silence and isolation due to fear of embarrassment.
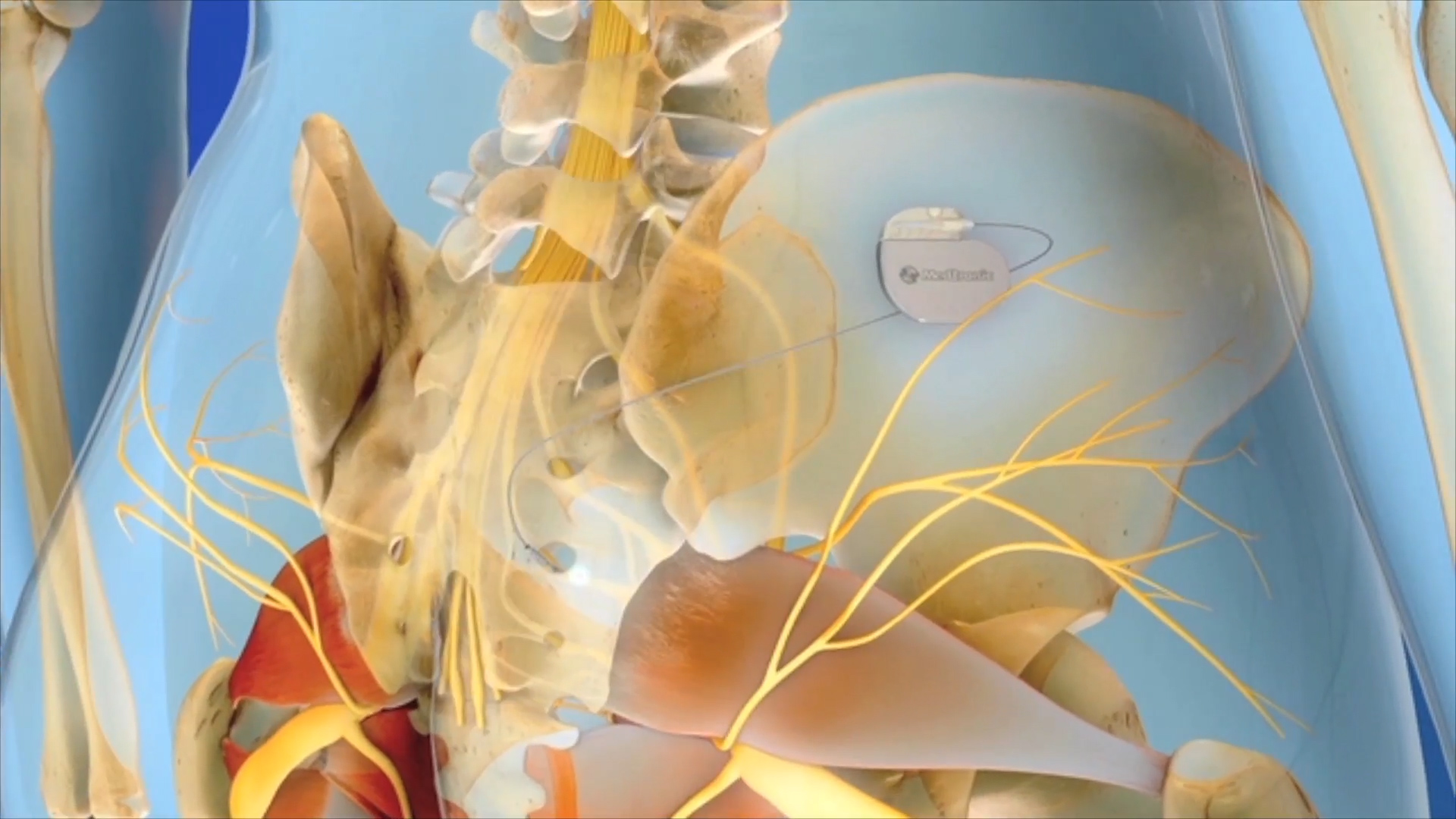
In the United States, there are 37 million people(1,2) suffering from overactive bladder, and up to 20 million(3,4) with fecal incontinence. For over 20 years, Medtronic has offered sacral neuromodulation (SNM) therapy. The InterStim™ device is small and fits in the palm of your hand. It is implanted in the patient near the upper buttock. The device is connected to a thin wire that stimulates the sacral nerve. The InterStim™ system addresses normalizing communication between the bladder, the bowel, and the brain.(5,6) For those who use the InterStim™ implant, 84 percent were satisfied with the therapy.7 Users also experience significant and sustained improvement in quality of life8 over those who use alternative treatments for—three times greater improvement in quality of life over those who use medications.9 That quality of life improvement means going to the bathroom less often and getting back to the activities they enjoy.
Despite the success of this therapy, a stigma exists around acknowledging these pelvic health issues. The InterStim™ device helps alleviate the symptoms associated with urinary and fecal incontinence. But Medtronic believed it could do more to help patients. Medtronic sought to provide more options to manage the implant in the hands of patients and to add a consumer-friendly management device that wouldn’t draw unwanted or unnecessary attention to the patient’s condition.
The Solution
An App-Controlled Implant That Fits Seamlessly Into Patients' Lives
The goal with improving the InterStim™ system sprang from a desire to make it fit better into a patient’s daily life. Earlier versions of the patient programmer looked like a garage door opener or a pager. “Finding a more discreet device was a major attractor of working with Samsung. Through our work together, we could leverage their smartphones as a platform to give patients the ability to adjust their implanted devices,” says Willenbring.
“The Samsung mobile device loaded with proprietary software applications is called the smart programmer for the InterStim™ system,” explains Lindsey Hanson, senior product manager for Pelvic Health and Gastric Therapies at Medtronic. “The smart programmer phone comes preloaded with the My Therapy application that lets patients check their stimulation level, adjust stimulation provided by the implant, turn stimulation off or on, switch stimulation levels, and change programs.”
“When a patient uses the smart programmer to adjust the therapy, all you see is someone holding what looks to be a typical mobile device, something so ubiquitous that no one questions its use,” says Hanson.

The introduction of the Samsung device occurs during the patient evaluation of the InterStim™ system. The evaluation allows a patient to test the therapy before physically implanting the device in their body. Temporary lead wires are inserted near the patient’s sacral nerve and connected to an external nerve stimulator. That evaluation period lasts anywhere from three days to two weeks. The physician adjusts initial settings, and the patient uses the same platform during the evaluation period to manage the therapy. If the evaluation goes well, the InterStim™ device is implanted and the patient uses the smart programmer.
“Patients can’t always anticipate when the symptoms of their condition will strike. There’s anxiety around getting caught in a bad and embarrassing situation,” says Hanson. “Not only does InterStim™ provide relief, but having the ability to control the treatment through the smart programmer offers them an extra layer of reassurance. The ability of the patient to manage their treatment is an important piece of therapy delivery.”
One Patient’s Story
Kirstie, an InterStim™ patient, had struggled with fecal incontinence for over 10 years before receiving sacral neuromodulation.
After receiving the InterStim™ implant, Kirstie has been able to gain back her quality of life. “The smart programmer has been so easy to use,” she explains. “With the guidance of my doctors, I am using the device to both change the programs and the amplitude settings within each program to find an optimal setting for my treatment.”
“[InterStim™ has] been a godsend. It allows me to start living my life again, get out of the house more, go out to dinner with my family.”
The Results
Consumer Technology Enhances Patient Experience
Adoption of the Samsung Galaxy smartphone as the platform for the smart programmer within the InterStim™ system was quick. “It took us roughly six months from launch to bring the smart programmer to nearly all our customer accounts,” reports Hanson. “Now new patients in the United States and most of Europe automatically receive the Samsung mobile device as part of the therapy system.”
In many ways, the Samsung smartphone has become the face of the InterStim™ therapy for patients, says Willenbring. “The patient knows the implant is there under their skin, but what they will see, day in and day out for literally years, is the Medtronic application user interface on that Samsung mobile device they hold in their hand,” he says.
Security was a critical consideration in designing the smart programmer. With defense-grade Samsung Knox security built in right down to the chip and a set of secure APIs to leverage, the device, application and patient data are highly protected.
The smart programmer allow patients to adjust their therapy in front of others without drawing attention to the fact that they have a pelvic health condition. Devices are user-friendly, and the smart programmer puts control into the hands of users.
An added benefit of using the My Therapy application on the Samsung smartphone is the ability to track patient behavior and interaction with the implanted device. Charts and graphs help patients and clinicians visualize when the program is on and active, and for how long. Logs show changes in stimulation programs and which programs were used most. Medtronic has opportunities to use that information in the future as it explores a Digital Health program to improve therapies offered and provide patients with more direction for managing their conditions. “Patients tend to normalize the effect of therapy and the resulting improvements. These graphs and charts make the treatment more tangible, helping patients connect the therapy to its impact on their lives,” Hanson explains.
“Medtronic is a leading global provider of medical devices, and Samsung is a leading global provider of consumer technology. “This matchup brings all of our strengths to the table,” says Willenbring. Offering those strengths in one treatment system has been life-altering for patients with pelvic health conditions. Willenbring sums up the extent of that impact, “These patients often suffer in silence and isolation until they seek treatment for their pelvic health conditions. Imagine the psychological challenge, the depression and anxiety that comes from leaving these conditions untreated. When InterStim™ is effective in these patients, it literally offers life-changing relief. It returns their quality of life.”
Citations
- Stewart WF, Van Rooyen JB, Cundiff GW, et al. World J Urol. 2003;20(6):327-336.
- United Nations, Department of Economic and Social Affairs, Population Division (2011). World Population Prospects: The 2010 Revision, CD-ROM Edition.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal Incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512-517.
- United States Quick Facts. United States Census Bureau Web site. Available at: https://www.census.gov/quickfacts/table/PST045215/00 Accessed July 19, 2016.
- Chancellor MB, Chartier-Kastler EJ. Principles of sacral nerve stimulation (SNS) for the treatment of bladder and urethral sphincter dysfunctions. Neuromodulation. 2000;3(1):15-26.
- Patton V, Wiklendt L, Arkwright JW, et al. The effect of sacral nerve stimulation on distal colonic motility in patients with fecal incontinence. Br J Surg. 2013;100:959–968.
- Foster RT Sr, et al., Neurourology Urodynamics. 2007
- Tjandra JJ, Chan MKY, Yeh CH, Murray-Green C. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494-502
- Siegel S, Noblett K, Mangel J, Griebling TL, Sutherland SE, et al. Results of a prospective, randomized, multicenter study evaluating sacral neuromodulation with InterStim® Therapy compared to standard medical therapy at 6-months in subjects with mild symptoms of overactive bladder. Neurourol Urodyn.
Indications for Use:
Sacral Neuromodulation delivered by the InterStim™ system for Urinary Control is indicated for the treatment of urinary retention and the symptoms of overactive bladder, including urinary urge incontinence and significant symptoms of urgency-frequency alone or in combination, in patients who have failed or could not tolerate more conservative treatments.
The following Warning applies only to Sacral Neuromodulation for Urinary Control:
Sacral Neuromodulation delivered by the InterStim™ system for Bowel Control is indicated for the treatment of chronic fecal incontinence in patients who have failed or are not candidates for more conservative treatments.
Contraindications for Urinary Control and for Bowel Control: Diathermy. Patients who have not demonstrated an appropriate response to test stimulation or are unable to operate the neurostimulator.
Warnings/Precautions/Adverse Events:
For Urinary Control: Safety and effectiveness have not been established for bilateral stimulation; pregnancy, unborn fetus, and delivery; pediatric use under the age of 16; or for patients with neurological disease origins.
For Bowel Control: Safety and effectiveness have not been established for bilateral stimulation; pregnancy, unborn fetus, and delivery; pediatric use under the age of 18; or for patients with progressive, systemic neurological diseases.
For Urinary Control and for Bowel Control: The system may be affected by or adversely affect cardiac devices, electrocautery, defibrillators, ultrasonic equipment, radiation therapy, MRI, theft detectors/ screening devices. Adverse events include pain at the implant sites, new pain, lead migration, infection, technical or device problems, adverse change in bowel or voiding function, and undesirable stimulation or sensations, including jolting or shock sensations. Patients should be assessed preoperatively for the risk of increased bleeding. For full prescribing information, please call Medtronic at 1-800-328-0810 and/or consult Medtronic’s website at www.medtronic.com. Product technical manual must be reviewed prior to use for detailed disclosure.
USA Rx Only. Rev 0517
ID number – UC202007726 EN